
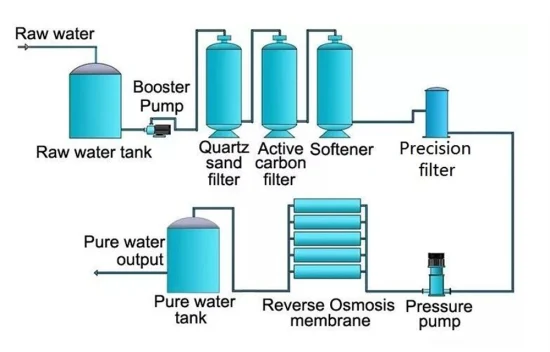
The process of removing salts and other inorganic ions dissolved in water is known as “demineralization”. Many scientific, medical, and industrial processes require purified water, so this is a common method to produce it. Membrane filtration and ion exchange resins are the usual tools for demineralization, which involves filtering out pollutants and unwanted minerals.
Key Concepts of Demineralization of Water
Emphasizing the removal of dissolved mineral ions to create water with a high level of purity is central to the demineralization process. When minerals are present, they can lead to problems like scale and corrosion; therefore, these processes are essential in many different sectors. The key concepts are as follows:
- Electrodeionization (EDI)
This is a continuous ion removal method that uses electrical currents in conjunction with ion exchange resins. The use of electrical currents to transport ions across membranes eliminates the need for chemical regeneration in this process. EDI is commonly employed in industries such as pharmaceuticals and electronics production. This is attributed to its ability to achieve exceptionally high levels of water purity.
- Water Quality Measurement
Electrical conductivity, or resistivity, is the standard way to determine the quality of demineralized water. A high resistance (or low conductivity) suggests that there are fewer dissolved ions since pure water does not transmit electricity very well. Water quality can also be evaluated by measuring Total Dissolved Solids (TDS).
- Mixed Bed Demineralization
A mixed bed unit is a vessel that combines anion and cation exchange resins. This enables much more comprehensive ion removal, leading to even more pure water. It typically serves as the final polishing step after a two-bed ion exchange system.
- Regeneration Process
Ion-exchange resins become ineffective after a certain amount of time because they become saturated with ions. A strong acid is required to restore cation resins, and a strong base is required to regenerate anion resins in order to restore their functionality. This procedure restores the resin’s ion exchange capacity by exchanging the undesirable ions for hydroxide and hydrogen ions.
- Reverse Osmosis (RO)
In reverse osmosis, pressure pushes water through a semi-permeable membrane. The membrane is permeable only to water molecules. This prevents the passage of any other contaminants, such as ions or dissolved salts. Combining reverse osmosis with ion exchange to increase water purity and decrease demineralizer load is common, although reverse osmosis is not usually considered demineralization on its own.
- Feed Water Pre-Treatment
In many cases, pre-treatment is required to safeguard the ion exchange system. Methods such as occasionally adjusting pH to maximize ion exchange efficiency, softening out hardness (calcium and magnesium), and filtering out suspended particles are all part of this process. When using EDI or RO, pre-treatment becomes even more crucial.
- Ion Exchange Process
- Strong Base and Strong Acid Resins
Because of their versatility in ion exchange, these resins find widespread application in demineralization and are capable of generating water of exceptional purity.
- Anion Exchange
An anion exchange resin is used to swap the following cations for hydroxide ions: bicarbonate (HCO₀⁻), sulfate (SO₄²⁻), chloride (Cl⁻), and nitrate (NO₀⁻). These are all negatively charged ions. Water (H2O) is formed when the resultant H⁺ and OH ions mix, thus eliminating salts from the water.
- Cation Exchange
Here, hydrogen ions (H⁺) are swapped out for positively charged ions (cations) in the water, such as potassium (K⁺), magnesium (Mg²⁺), calcium (Ca²⁺), and sodium (Na⁺). A cation exchange resin is utilized for this purpose.

By manipulating these critical parameters according to the requirements of the application, demineralization can produce water of different purity levels.
Benefits of Demineralization of Water
Demineralization, also known as deionization, is a method for purifying water by eliminating salts and minerals. The following are some of the many fields and uses for which this method is useful:
- Cosmetic Industry
Skincare and cosmetic goods frequently use demineralized water as a basis to prevent mineral pollutants from changing the product’s qualities or irritating the skin.
- Cleaning and Car Wash Industries
The fact that demineralized water dries without leaving behind residues or mineral stains makes it a popular choice for window cleaning and automobile washes.
- Medical Use and Pharmaceutical
Pharmaceutical production and medical settings use demineralized water to prevent ions and minerals from disrupting these environments.
Laboratory tests, autoclaves, and dialysis machines all use demineralized water to eliminate contaminants that could damage delicate machinery or compromise results.
- Electronics Manufacturing
Cleaning and manufacturing operations involving electronic components (such as semiconductors) require demineralized water. This is to ensure the components are free of flaws or short circuits due to minerals.
Electronic devices can also benefit from mineral-free water’s improved heat transmission and reduced likelihood of damage when utilized for cooling purposes.
- Power Generation
Demineralized water is essential for power plants, particularly those that use steam turbines. This is because it keeps mineral scales from accumulating and guarantees safe and efficient operation.
In the power generation sector, demineralization plays a significant role in controlling conductivity and preventing scaling in electricity production.
- Lab Use
If you want your results from a biological or chemical experiment to be accurate and repeatable, you need to use pure water to prevent reactions or interference from trace minerals.
- Industrial Applications
Cooling towers, boilers, and other machinery can endure less wear and tear and maintenance expenses thanks to demineralized water, which stops mineral deposits and scale from forming. For instance, turbines and steam boilers run more efficiently and with less power when there are no mineral deposits in the way.
Demineralized water protects pipelines and other metal surfaces from corrosion by removing ions that cause oxidation and rust.
- The Food and Beverage Industry
Demineralized water ensures the absence of minerals in processed foods, soft drinks, and bottled water. This makes them taste better and last longer.
The procedure ionizes the water to remove heavy metals like arsenic and lead, ensuring it is safe to drink.
Disadvantages of Not Demineralizing Water
Depending on the application, there are a number of drawbacks to not demineralizing water. Here are a few major drawbacks to consider:
- Health Concerns in Certain Uses
While it’s true that some water minerals are beneficial for you, it’s also true that minerals like copper, iron, and lead can be dangerous in high doses. This becomes particularly concerning when people use the water for cooking or drinking without adequate supervision.
- Spots and Stains
When used for rinsing or washing, the mineral deposits in untreated water might stain or leave spots on glassware, dishes, and surfaces.
- Decreased Effectiveness of Water Treatment Methods
Ion exchange resins, RO membranes, and water softeners may not work as well with hard water, which is water with a high mineral concentration. More regular cleaning or component replacement could be necessary as a result, which would increase operational costs.
- Taste and Aesthetic Problems
A metallic aftertaste or discoloration in drinking water might be due to a high mineral concentration. Customers find this unpleasant, but businesses that depend on clean water, such as those producing food and drinks, find it particularly problematic.
- Detergents and Soaps’ Ineffectiveness
Hard water’s mineral content makes detergent and soap less effective since they react with one another. Soap scum can form on skin, clothing, and surfaces, and detergent usage increases as a result.
- Equipment Scaling and Corrosion
Scaling can occur in sectors that utilize machinery, such as cooling towers and boilers. This is due to untreated water containing minerals such as iron, magnesium, and calcium. The buildup of scale in pipes and heat exchangers causes them to work less efficiently, leading to higher energy bills and more frequent maintenance requirements.
It’s possible for certain minerals to encourage corrosion in equipment and pipes, which can cause expensive breakdowns and repairs.
- Interference with Chemical Reactions
Minerals can hinder chemical reactions, leading to contamination or incorrect outcomes in processes where water purity is crucial, such as in laboratory settings and pharmaceutical industries.
Because of these problems, demineralization is crucial, especially for procedures or industries that demand very pure water.
Conclusion
Demineralized water is an indispensable resource for businesses that need a high level of consistency, accuracy, or cleanliness. It provides numerous advantages, including the improvement of safety, protection of machinery, and the enhancement of product quality.


 Benefits of Air Admittance Valves
Benefits of Air Admittance Valves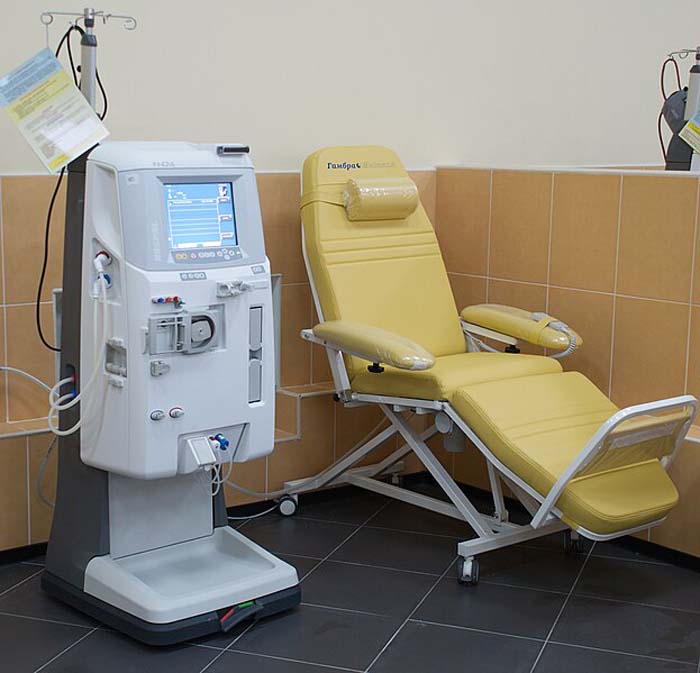




 When applied to metal surfaces, hydrogen peroxide can aid in the breakdown of organic deposits. This allows other CIP chemicals to access and dissolve them more easily. When compared to other CIP chemicals, hydrogen peroxide often needs less time in contact with the metal surface while still effectively penetrating deep deposits. During inactivity, it shields against corrosion and effectively stops the deposition of new sediments.
When applied to metal surfaces, hydrogen peroxide can aid in the breakdown of organic deposits. This allows other CIP chemicals to access and dissolve them more easily. When compared to other CIP chemicals, hydrogen peroxide often needs less time in contact with the metal surface while still effectively penetrating deep deposits. During inactivity, it shields against corrosion and effectively stops the deposition of new sediments.
 Forward osmosis
Forward osmosis 


 The difference between UF and NF filtration?
The difference between UF and NF filtration?